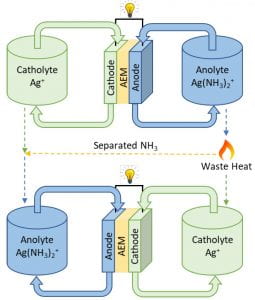
 We must reduce our dependence on fossil fuels in the near future if we are going to combat climate change. There are many challenges to this, but energy storage technologies will be key to the optimal operation of a future electrical grid supplied by diverse energy sources. Thermally-regenerated batteries (one is shown schematically to the left) are electrochemical energy storage devices that have the potential to not only store excess electricity, but to also provide a means to convert waste heat into electrical energy for distribution through the grid. This means that low-grade waste heat from industrial sources may become a future source of additional electrical power.
We must reduce our dependence on fossil fuels in the near future if we are going to combat climate change. There are many challenges to this, but energy storage technologies will be key to the optimal operation of a future electrical grid supplied by diverse energy sources. Thermally-regenerated batteries (one is shown schematically to the left) are electrochemical energy storage devices that have the potential to not only store excess electricity, but to also provide a means to convert waste heat into electrical energy for distribution through the grid. This means that low-grade waste heat from industrial sources may become a future source of additional electrical power.
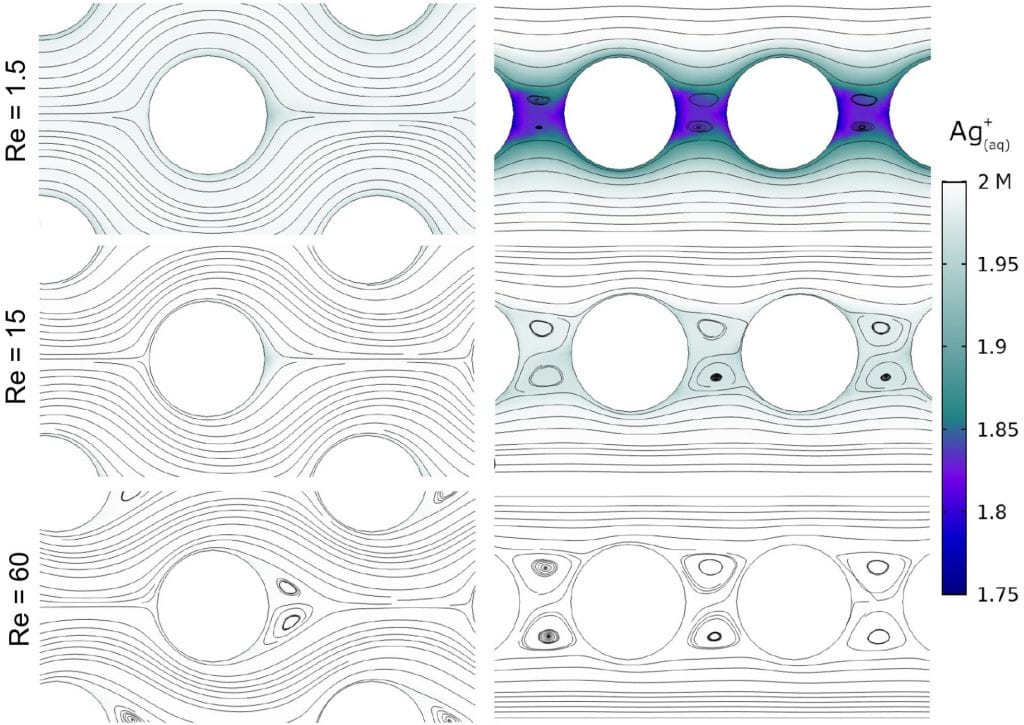
These batteries are not yet economically feasible, so we are investigating ways to improve their performance and increase their implementation feasibility through enhanced heat and mass transport. We are simulating the electrochemical power output of the flow cell of this battery using multiphysics simulations in Comsol. Typically, these batteries are discharged by flowing electrolytes through a small reaction cell, shown schematically above. The reaction cell consists of porous electrode materials (e.g. carbon felt) and an ion exchange membrane to separate the anode and cathode. We have shown that the structure of the electrode can have a large influence on the battery power output as the fluid flow through the electrode controls the reactant concentrations at the electrode surfaces. Below, we show how an electrode structured as a staggered array of fibers (left column) maintains high concentrations of the reactant (Ag in this case) at all Reynolds numbers we investigated. In contrast, an in-line fiber arrangement (right column) creates reactant dead zones between electrode fibers at low Reynolds numbers. High fluid flow velocities (i.e. high Re) must be used to compensate and maintain similar power output compared to the staggered fiber arrangement.